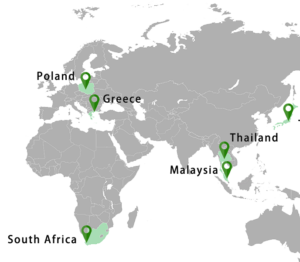
ギリシャに本社を持つカンスングループは、ヨーロッパ全土を中心に世界21カ国に薬局11,000店舗、医師35,000名と連携し、商品を展開しております。
ギリシャ及び南アフリカに政府認可の医療大麻栽培場(約26ha)及び抽出設備を有し、世界初となる原料栽培からGMP設備での製造までを実現しております。
主な取扱い商品は、カンナビノイド製品群(CBD製品)や植物性生理活性物質を主成分とする医療用医薬品、一般用医薬品、化粧品及びサプリメントです。

The Cannsun Group develops products in collaboration with 11,000 pharmacies and 35,000 doctors in 21 countries around the world, mainly throughout Europe.
We have government-approved medical cannabis cultivation plants (about 26ha) and extraction facilities in Greece and South Africa, and we have realized the world’s first raw material cultivation to manufacturing with GMP facilities.
The main products we handle are cannabinoid products (CBD products), ethical drugs, over-the-counter drugs, cosmetics and supplements that are mainly composed of plant-derived physiologically active substances.
2021年、厚生労働省が日本国内でのCBDを含む製品の販売及び使用に関する検討を進めています。
一方、
In 2021, the Ministry of Health, Labor and Welfare is studying the sale and use of products containing CBD in Japan.
On the other hand, in Europe and the United States, CBD products have already been used in healthy people and patients with official approval.
CANNSUN JAPANは、カンスングループの日本をはじめとするアジア事業を推進する関連会社であり、CDBを含む製品の規制に関する国内での討
また、規制要件を踏まえたうえで、CDB製品の

CANNSUN JAPAN is an affiliated company that promotes Cannsun Group’s business in Japan and other Asian countries. We Informs consumers of discussions in Japan regarding regulations on products including CDB. In addition, we will develop CDB products in Japan based on regulatory requirements.